
Since Dr. Ancel Keys and his colleagues formulated and promulgated the diet-heart hypothesis in the 1960's, the idea of eating saturated fat has become anathema in most nutritional circles. When Americans were told that that consumption of saturated fat was positively correlated with the incidence of heart disease, they began to eat more of the "heart-healthy" mono- and polyunsaturated fats and fewer of the saturated ones. In spite of that, the number of hospital discharges with cardiovascular disease as the first listed diagnosis has continued to increase in the U.S. This is especially surprising in light of the fact that the percentage of U.S. adults who smoke has declined from over 40% in 1965 to about 20% in 2007. Is it possible that saturated fats are not as evil as they have been portrayed?
To begin the discussion, it is important to understand that a saturated fat is not saturated with calories or with cholesterol. In this case, "saturated" is a chemical term, and it means that the molecule in question is saturated with hydrogens--that is, it contains the maximum number of hydrogens it can hold. Here are two fatty acids, one saturated and the other unsaturated:

In the fatty acid at the top, the carbon-carbon bond between the two green C's is a single bond. Each green carbon holds two hydrogens, and they are saturated with hydrogen. In the fatty acid at the bottom, there is a double bond between the two green C's. Each of those carbons holds one hydrogen. The carbons do not hold as many hydrogens as they possibly could and they are therefore unsaturated. This particular fatty acid has only one unsaturated carbon-carbon bond, so it is monounsaturated. If it had two or more unsaturated bonds, it would be polyunsaturated.
The important thing about unsaturated fatty acids is that the presence of a double bond weakens the carbon-hydrogen bonds on the carbons next to the double bond. In the picture above, those carbon-hydrogen bonds are marked with green asterisks. That doesn't sound particularly interesting until we understand what happens when those hydrogens are removed by something like oxygen, heat or metal ions. As soon as we remove one of the vulnerable hydrogens, our heart-healthy unsaturated fatty acid becomes a free radical. In other words, it contains an unpaired electron and it becomes extremely chemically reactive.
Once the first free radical is formed, the generation of free radicals from unsaturated fatty acids happens in a self-propagating manner. One free radical can interact with other unsaturated fatty acids to produce more free radicals, which in turn produce even more free radicals, and so on. Besides damaging the fatty acids, these free radicals can also destroy other molecules, including vitamins and proteins. In addition, the free radicals are able to react with oxygen to produce hydroperoxides. These eventually break down into aldehydes, which produce the odors and flavors associated with rancidity.
The reactivity of fatty acids increases with the number of double bonds they contain. Stearic acid is an 18-carbon saturated fatty acid. If we add one double bond, it becomes one hundred times more likely to form a free radical. If we add three double bonds, it becomes 2500 times more likely to form a free radical. The health effects of saturated versus unsaturated fatty acids won't be addressed until the next blogpost, but it is certain that saturated fatty acids are far more stable than their unsaturated counterparts.
There are several ways to decrease the likelihood of free radical formation and rancidification in fatty acids. One is to be sure that heat is not used to extract the fatty acid from its source. In the case of unrendered animal fats, this is not a problem. In the case of vegetable fats, cold pressing ensures (at least it does in the EU) that the oil will not be heated above about 80 degrees Fahrenheit. Unfortunately the U.S. definition of cold pressed is not particularly rigorous, so it may be necessary to check websites or make telephone calls to the manufacturer to determine the temperature a particular brand of oil reaches as it is extracted. When fat is used for cooking, it is important to realize that the higher it is heated and the longer it is heated, the more likely it will be to form free radicals.
Another strategy to avoid free radical formation and rancidification in fats and oils is to be sure that they are kept away from light, particularly UV light. It is also helpful to keep fats and oils away from oxygen. They should not be stored for long periods, and once a container is opened, it should be used up as quickly as possible.
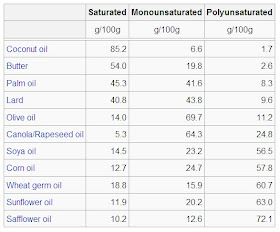
As we have already seen, some polyunsaturated fats are necessary for growth and for optimal health. However it pays to know which fats are which and to be careful with respect to the amounts and types of dietary fats we consume. In closing, here is a table that presents the approximate composition of some common fats, arranged from the lowest to the highest percentage of polyunsaturated fatty acids.
You don't post very often, but your posts are always informative. Thank you making nutritional biochemistry comprehensible to ordinary folks like me.
ReplyDeleteThanks, whatsonthemenu! I love biochemistry, and I'm always glad when I see that other people appreciate it, too!
ReplyDeleteGreat post. Your last post had me questioning my knowledge of the differnces between mono, poly, and saturated fats. Knowing that one is better than the other and being able to explain, or know why are two entirely different bases of understanding. Keep up the good work.
ReplyDeleteStormyCatalyst
Thanks, StormyCatalyst! Perhaps the stick diagrams were confusing rather than helpful. Anyway,
ReplyDeleteSaturated=no double bonds
Monounsaturated=one double bond
Polyunsaturated=more than one double bond
The more double bonds, the more unstable the oil or fat will be in the presence of oxygen and/or heat.
Omega-3's and omega-6's have lots of double bonds and are very unstable, but fortunately we only need a small amount of each of them every day in order to stay healthy.
Do you read Dr. William Davis' Heartscan blog? What do you make of his latest post on butter, insulin rises, and fat storage? I and other posters wonder if the problem is
ReplyDeletea) the combination of butter and wheat carbs and not just butter alone - in other words, would a meal combo of butter, meat, and nonstarch veggies cause the same insulin spike?); and
b) the milk solids in butter - does whey and casein-free clarified butter cause the same insulin surge?
I do think dairy products like milk, yogurt, and cheese with mixed macronutrients can be a problem, but I wonder about clarified butter versus other saturated fats.
I do read Dr. Davis's Heart Scan Blog, and like you I was puzzled by his comments on butter. (For those who haven't seen his blogpost on butter, it is here.)
ReplyDeleteThe study he referenced can be found here in PDF form. I'm sorry to admit it, but the study puzzles me. The patients ate a fat-rich meal consisting of dietary fat [50 g/m2 body surface area of butter, refined olive oil (ROO), high-palmitic sunflower oil (HPSO) or a mixture of vegetable and fish oils (VEFO)] along with a portion of plain pasta (30 g/m2 body surface area), one slice of brown bread, and one container of skim yogurt. The control group ate only the pasta, bread and skim yogurt, a meal which was low in calories but relatively high in carbs.
The control group didn't show much of a glucose spike or much of an insulin spike at one hour, but when the fat was added, the glucose and insulin spikes were much higher and also occurred at one hour.
That doesn't make any sense to me. Normally the addition of fat slows digestion, but it didn't in this case. When fat is eaten by itself it does not cause the release of insulin, but in this case, adding it to carbs caused significantly more insulin to be released. Would the same thing have happened if the fats had been added to a meal of lean beef and broccoli? I don't know. Another consideration is that ketoadapted low-carbers will not have the same insulin response to carbs, proteins and fats as compared to people who spend their lives eating the Standard American Diet. (See Induction Flu for a brief explanation.)
I have been reading the Heart Scan comments section to try to gain some more insight into the problem and will add more information here if I find anything that might add to the discussion.
For another perspective on the butter question, here is an interesting explanation of lipolysis, chylomicrons and FAT STORAGE ON YOUR BUTT at Peter's Hyperlipid blog.
ReplyDeleteI read Peter's post as well as dissenting comments by people better able than me to pick apart the study Dr. Davis cited. I don't think I'll be scratching butter off my farmers' market shopping list.
ReplyDeleteDr. Davis used to be a vegetarian, and that seems to color his perspective on which foods are healthy and unhealthy. I'm glad he is starting to discuss the science of low-carb with his readers. There seems to be a mutual benefit as he comes to the discussion from a clinical perspective and the readers are more informed on the basic science.
ReplyDeleteI'm with you. Until I get more convincing evidence, I'll be keeping butter on my menu.
Was just reading your glucose, glycogen and gluconeogenesis post-so I hope I can ask a question here not related to satfat (at least directly). While eating low carb, with very low insulin, what is the hormonal signal to turn GNG glucose into glycogen? It seems most signals in this state would be toward glycogen breakdown (adrenaline, glucagon). If you know of a resource that helps answer this (I haven't been able to find much on the mechanisms for glycogen generation with low insulin) that would be great. Thanks and nice blog.
ReplyDeleteAs you know, insulin is secreted when protein is ingested, even if zero carbs are eaten with it. The level of insulin secretion is lower, gram for gram, than it would be for carbohydrate, and glucagon is also released to prevent blood sugar from going too low.
ReplyDeleteThat said, I have to admit that I don't know a lot about how glycogen content is regulated. I would assume that the body is able to sense how much glycogen it needs to keep in storage, both in the muscles and in the liver. Presumably that would be different depending on the athletic conditioning of an individual and on whether he or she relied predominately on carbs or on lipids for energy. But how the set-point is determined, I can't say. (Any readers with any information in this area, please chime in.)
I would guess that in the case of glycogen depletion, the body would upregulate the insulin sensitivity of the glycogen synthesis pathway and cause sugar from gluconeogenesis to be shunted in that direction until glycogen stores had been replaced. But that's only speculation on my part. In a brief Googlesearch I couldn't come up with a specific signaling mechanism for the regulation of glycogen content in the context of low insulin and relatively low blood sugar.
Thanks for the reply-I guess I'm glad I'm not the only one who doesn't know this-I've been looking into it a bit and haven't found much other than GNG can help restore glycogen. That's about it. Anyway, I appreciate the response.
ReplyDeleteYou're welcome. Yes, if few carbs are eaten, the glucose must come from gluconeogenesis. And I couldn't find a hormone besides insulin that promotes glycogen storage. I found interesting references that showed that both mice and humans are able to live without the ability to synthesize liver glycogen. It's not optimal, but they can survive. If you look at the symptoms these mice and humans experience, it becomes obvious that even zero-carbers with very low insulin secretion are synthesizing and utilizing at least some liver glycogen.
ReplyDeleteNice post. I happened to make a YouTube video about this subject http://www.youtube.com/watch?v=3GYQ-m9fU9w. VBR Hans Keer
ReplyDeleteThanks for sharing that, Hans! It's a good summary of the important points about polyunsaturates.
ReplyDelete